

"Genetron Health's oncology platform covers multiple cancers. Our key capabilities include by detecting unique gene mutations in patients, assisting patients in selecting targeted drugs and chemotherapeutics, evaluating the efficacy of immunotherapy, dynamically monitoring the progress of drug resistance and other diseases treatment, and assisting in formulating and optimizing clinical trials treatment plan to achieve personalized diagnosis and treatment."








01
Next-Generation
Sequencing Method

Next-Generation
Sequencing Method
Next-Generation
Sequencing Method
Next-Generation
Sequencing Method

PCR-Fluorescence
Probe Method
PCR-Fluorescence
Probe Method
PCR-Fluorescence
Probe Method

PCR-Fluorescence
Probe Method

BIOCHIP READER
DIGITAL PCR SYSTEM

HIGH THROUGHPUT
SEQUENCING SYSTEM

FULLY AUTOMATIC
SAMPLE FEEDING SYSTEM

HIGH THROUGHPUT
SEQUENCING SYSTEM
02

Chip-based digital PCR system
Perfect quantification: Approved by the NMPA, with a detection sensitivity reaching 0.1%.
Flexible throughput: single sample detection, multi-item combination, rapid application.
Convenient operations: manual operations for the whole process require less than five minutes; from sample preparation to report generation, a full cycle can be completed in only four hours.
Sealed system: After the chip is prepared, the whole system is sealed to reduce contamination risks.
Wide range of applications: it can be used for quantitative detection of rare mutations, copy number variation detection, methylation detection, and viral load detection; this is an open platform that can support a variety of detection kits.

GENETRON S5 is a new generation of clinical-grade semiconductor desktop sequencing platform, and is a reliable solution for local operations that require medium/low throughput.
Convenient operations and efficient sequencing: manual operations do not exceed 45 minutes, rapid sequencing can be done in 2.5 hours, and results can come out in two days.
Flexible throughput: It can be adapted to five different data volume chips, providing sequencing read lengths of 200bp, 400bp, and 600bp.
Low starting volumes: it can achieve as low as 10ng nucleic acid starting amount for library construction and sequencing.
Smooth data analysis process: The built-in Chinese version of the GENETRON S5 Server system is more convenient for data quality control and analysis, and it can quickly interpret data when used in conjunction with Genetron's automated reporting system.
Comprehensive application: applicable to a variety of different detection fields (tumor gene detection, genetic disease detection, pathogenic microorganism detection, metagenomic analysis, etc.).

GENETRON S2000 is a mid-to-high-throughput sequencing platform that is based on combined probe anchored polymerization (cPAS) technology.
High-quality data: DNB is prepared by rolling circle amplification technology to restore original genome information; sequencing data has a lower repetition rate; low tag jumps.
Flexible throughput: medium/high-throughput sequencing chips; single/dual-chip sequencing mode; multiple sequencing read length strategies to flexibly meet the needs of medium and high throughput sequencing.
Convenient and easy to use: manual/automatic chip loading mode; ready-to-install modular sequencing reagent consumables, with a visualized rapid analysis system, simple and efficient.
Wide range of applications: suitable for capture sequencing, whole exome sequencing, whole genome sequencing, etc.; widely used in clinical and scientific research fields (tumors, genetic diseases, reproductive health, pathogenic microorganism detection, etc.).

GENETRON Chef is a fully automated, high-throughput sequencing, library construction and chip loading preparation system, providing fully automated, unsupervised high-throughput sequencing pre-processing solutions.
Efficient and stable operating platform: It can deliver various library preparation operations, template preparation and chip loading, and ensure the accuracy and efficiency of the experiment.
Fully automatic workflow: It only takes 15 minutes of manual operations to complete preparatory work before the start of the automated workflow, without any additional manual intervention required during the process.
Compatible with multiple sequencing platforms: supports GENETRON S5 system, Ion GeneStudio S5 series, Ion Proton system and Ion PGM system.

The human IDH1 gene mutation detection kit can detect IDH1 R132H mutations contained in the tumor tissue DNA of glioma patients, providing them with vital pathological typing and prognostic evaluation reference information.
High sensitivity: can detect as low as 1% of gene mutations in 10ng/ul sample sizes.
Strong specificity: up to 99% or more.
Easy interpretation of results: The results are delivered as data, requiring no subjective analysis.
Convenient operations: 90 samples can be tested on the machine at a time, requiring just one hour to complete. The fully enclosed system reduces pollution.

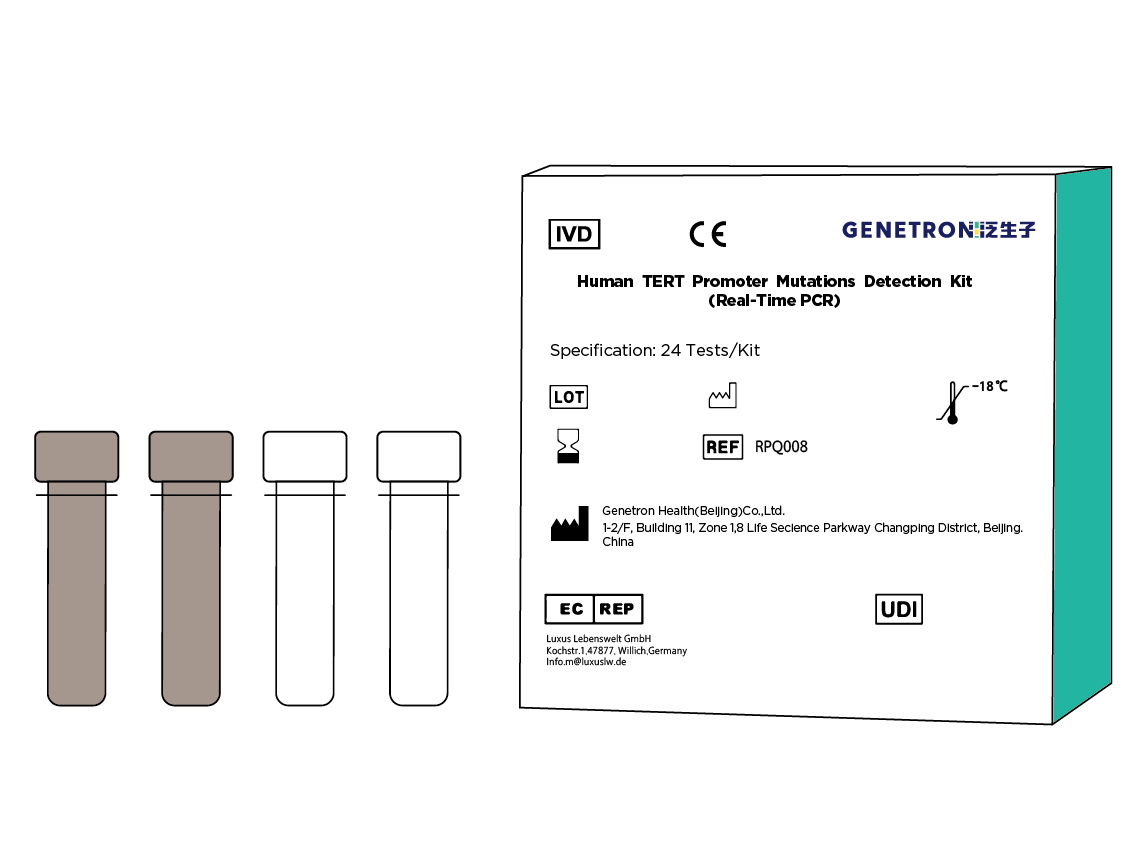
Human TERT gene promoter mutation detection kit (PCR-fluorescent probe method) The human TERT gene promoter mutation detection kit can detect the TERT C228T and C250T promoter mutations contained in the tumor tissue DNA of glioma patients, providing vital pathological typing and prognostic evaluation reference information.
High sensitivity: can detect as low as 1% of gene mutations in 10ng/ul sample sizes.
Strong specificity: up to 99% or more.
Easy interpretation of results: The results are produced as data, without need for subjective judgment.
Convenient operations: 90 samples can be tested on the machine at a time, and tests can be completed in just one hour. The fully enclosed system reduces contamination.

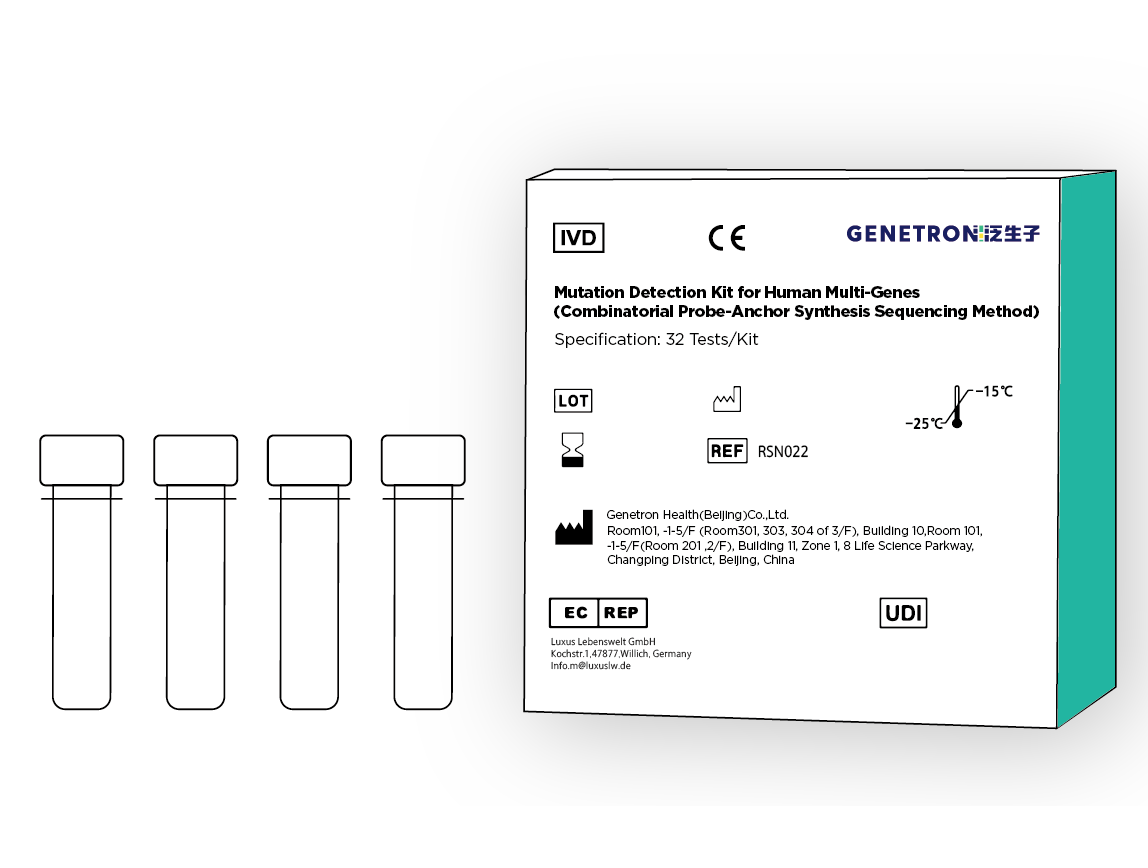
This kit is based on Genetron Health's patented "One-Step Method" technology ; library construction can be completed in 1.5 hours.
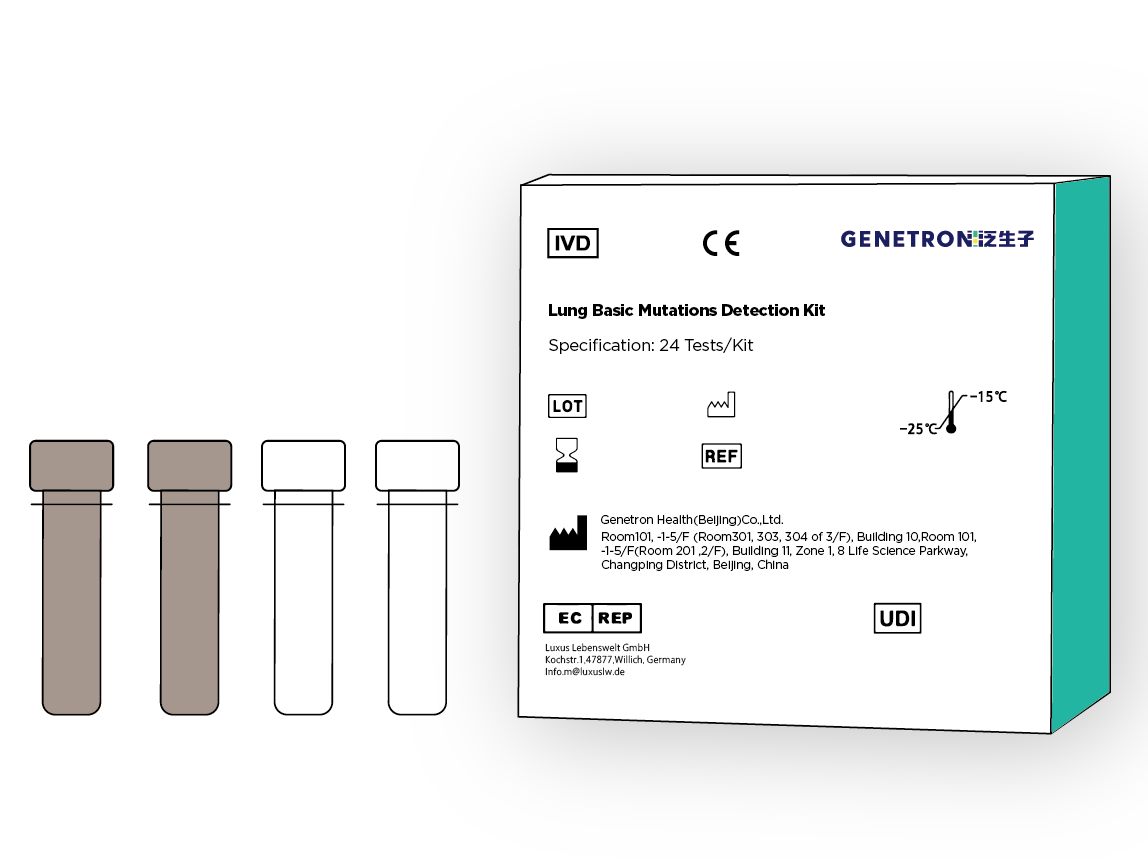
Qualitative detection of EGFR, KRAS, BRAF, HER2 and PIK3CA gene mutations in DNA, in formalin-fixed paraffin-embedded sections (FFPE) samples of tumor tissues diagnosed as non-small cell lung cancer (NSCLC) patients, and ALK in RNA Fusion with ROS1 gene and skipping of exon 14 of MET gene.

Novel coronavirus (2019-nCoV) nucleic acid detection kit (fluorescence PCR method).
Aboundant and Reliable—simultaneous detection of ORF1a/b gene sequence and N genes; introduction of internal reference controls; minimum detection with just 10 copies.
Anti-pollution system—Introduce dUTP and UDG enzymes to prevent contamination.
Simple & fast—10 min manual operations, detection can be finished in 2.5 hours, suitable for large-scale rapid detection.
Primary lung cancer (PLC) is one of the most common malignant cancers worldwide. Due to air pollution from industrialization and urbanization, along with the high smoking rate in China, the incidence and mortality of lung cancer have been on the rise. From the perspective of pathology and treatments, there are two main classifications of lung cancer: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). About 80% to 85% of lung cancers are NSCLC, while the rest are SCLC. Since the SCLC is characterized by unique biological behavior, the combined treatment of chemotherapy and radiation therapy is mainly used on patients, except for a few early-stage cases. The "lung cancer" herein refers to "NSCLC", unless specified. Systemic treatments for advanced NSCLC include chemotherapy, small molecular targeted therapy, immunotherapy, and anti-angiogenesis drugs. Most lung cancer cases at the early stages tend to have no distinct symptoms and patients are mostly diagnosed in advanced stages, resulting in average five-year survival rates of only 16%.
References:
Chinese Expert Consensus on Immune Checkpoint Inhibitors for Non-small Cell Lung Cancer
(2019 Version).
Brain tumors include primary brain tumors and brain metastases.
Common primary brain tumors include gliomas, meningiomas, pituitary tumors, ependymomas, embryonal tumors, etc. Common brain metastases include lung cancer brain metastases, and breast cancer brain metastases, melanoma brain metastases, etc.
Genetic testing can provide brain tumor patients with molecular diagnostics & prognostic evaluation, screening for targeted immunotherapy drug solutions, assessments for chemotherapy sensitivity, optimizing targeted treatment plans for diagnosis while also providing a hereditary genetic risk profile.

Malignant liver tumors are divided into two main classifications: primary and secondary. Primary hepatic cancer has high incidence and mortality, due to the large population of hepatitis B patients in China. Liver cancer is mostly generated from post-hepatitic cirrhosis in hepatitis B and hepatitis C patients. Primary hepatic cancer has insidious onset, inconspicuous early symptoms, rapid progression, and high malignancy. Most of these diseases have already advanced when diagnosed, and the best window of time for treatment has passed, so the five-year survival rate is extremely low. At present, surgical resection is the main radical treatment for primary hepatic cancer, with a five-year survival rate of only about 50% and a five-year recurrence rate of 60%–70%. The clinical treatments for advanced primary liver cancer consist of mostly systemic drug therapy, including systemic chemotherapy, molecular targeted therapy, and immunotherapy. Pathological types of primary liver cancer include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and mixed type of HCC-CC. HCC is the most common (85%-90%), followed by ICC (10-15%), and the mixed type of HCC-CC is the least common. “Liver cancer” often refers to "hepatocellular carcinoma".
References
Guidelines of Chinese Society of Clinical Oncology (CSCO), Hepatocellular Carcinoma
(2020).

Hematological cancer are malignant tumors that occur in the blood system (bone marrow, hematopoietic and lymphatic tissues). The most common hematological tumors are various types of leukemia, multiple myeloma and malignant lymphoma.
Leukemia is the most common hematological tumor, caused by malignant transformation of white blood cells, and is classified as acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia/chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL) and rare types of leukemia.
Multiple myeloma (MM) is a malignant plasma cell hematological tumor that occurs mainly in middle-aged and elderly people, MM incidence accounts for approximately 10% of hematological tumors [1], and is the second most common hematological malignancy after malignant lymphoma in many countries.
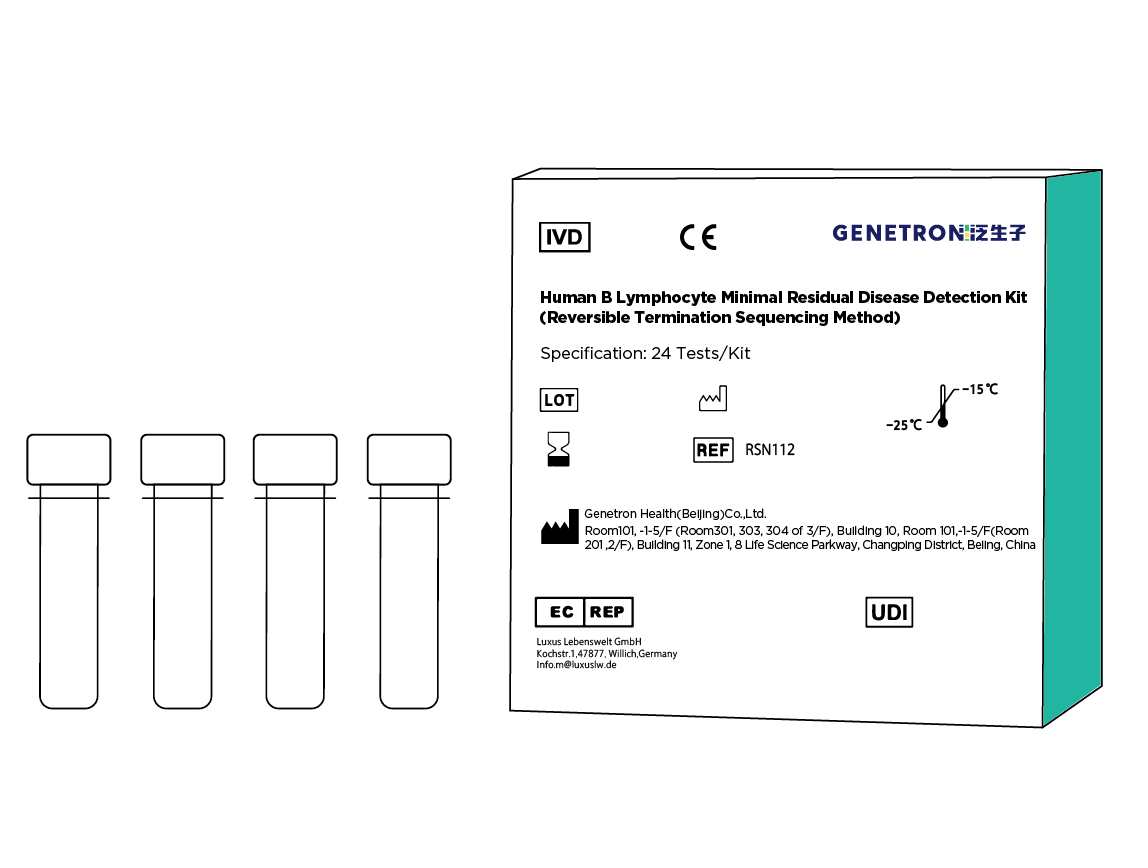
Treatment of hematological tumors generally include drug therapy, surgery, chemotherapy, immunotherapy and hematopoietic stem cell transplantation. After remission of hematological tumors, a small number of tumor cells remain in the body, which is the root cause of recurrence of the disease. Minimal Residual Disease (MRD) testing can provide early prediction of recurrence, stratify the patient's risk level, guide post-remission treatment, and determine the optimal time for bone marrow and stem cell transplantation. MRD testing includes four methods: Flow cytometry, quantitative RT-PCR of fusion genes, quantitative PCR of IgH/TCR rearrangements, and NGS sequencing. For hematological tumors such as multiple myeloma (MM), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), NCCN guidelines recommend the use of flow cytometry and NGS for MRD detection. [2] [3] [4] [5]
References:
[1] Milad Moloudizargari. The emerging role of exosomes in multiple myeloma[EB/OL]. [November 2019]. https://www.sciencedirect.com/science/article/abs/pii/S0268960X19300529.
[2] NCCN Clinical Practice Guidelines for Acute Lymphoblastic Leukaemia.
[3] Clinical Practice Guidelines for Acute Lymphoblastic Leukaemia in Children
[4] Guidelines for the diagnosis and treatment of multiple myeloma in China
[5] NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma

Gastrointestinal tumor are common malignant tumors in the digestive system, including gastric cancer, colon and rectal cancer. About 30% of gastric cancer patients in China have advanced gastric cancer with distant metastases, and their 5-year survival rate is less than 10%, while the survival rate of locally progressive gastric cancer is 13%-34% [1]. Gastric cancer can be divided into various types, among which adenocarcinoma is the most common, accounting for more than 90% [2]. The liver is the most observed target organ for hematogenous metastases [3].
At present, treatment of advanced gastric carcinoma includes systemic chemotherapy, surgical resection, radiation therapy, targeted therapy, and immunotherapy. Although the treatment methods are constantly optimized, the median survival times of patients is still less than a year. In addition, HER2 overexpression is detected in only about 16% of gastric adenocarcinoma patients[4], and the use of first-line therapeutic targeted agents is low.
Colorectal cancer (colon cancer), is also a common malignant tumor in the digestive system, including colon and rectal cancer. The most common type of colorectal cancer is adenocarcinoma, which can metastasize through blood and lymphatic systems. The five-year survival rate of patients with early-stage colorectal cancer reaches 80%–90%[5], but 50%–60% of the patients tend to already be in advanced stages when diagnosed[6], with livers and lungs as common metastatic sites. Major treatments for colorectal cancer include targeted therapy, immunotherapy, and chemotherapy. Genetic testing provides guidance for medication selection and also provides genetic risk information. The testing of the following genes is recommended for colorectal cancer patients, under NCCN guidelines: KRAS/NRAS/BRAF/NTRK/HER2/MSI/MMR[7].
References:
[1] Zheng XH, Xie YB. Diagnosis and Treatment of Advanced Gastric Cancer in China [J]. Oncology Progress, 2019, 17(1): 13–19.
[2] Lisa Hazard, John O'Connor, Courtney Scaife. Role of radiation therapy in gastric adenocarcinoma[J]. World J Gastroenterol, 2006, (10).
[3] Wang, Liwei, Chen, etc. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Gastroesophageal Juncyion[J]. 2016, 34 (13).
[4] Chinese Expert Consensus on Molecular targeted Therapy for HER2 Positive Advanced Gastic Cancer (2016 Version).
[5] Cancer Facts & Figures 2020|American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 14 July 2020).
[6] García-Alfonso et al., Bevacizumab in combination with biweekly capecitabine and irinotecan, as first-line treatment for patients with metastatic colorectal cancer, British Journal of Cancer.2010,103:1524 – 1528.
[7] NCCN Guidelines Version 2.2023 Colon Cancer.

Thyroid cancer is a malignant tumor that originates from the follicular or
parafollicular epithelial cells of the thyroid glands, which is also the most common
malignant tumor in the head and neck regions. In recent years, the incidence of thyroid
cancer has increased rapidly worldwide. According to the National Center of Cancer
Registry, the incidence of thyroid cancer ranks fourth in women in urban areas of China,
among all malignant tumors in women. The number of thyroid cancer cases in China will
continue to increase at a rate of 20% per year.
According to the tumor origin and differentiation, thyroid cancer is further classified
into papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary
thyroid carcinoma (MTC), and anaplastic thyroid cancer (ATC). PTC is the most common
type of thyroid cancer, accounting for about 85%–90% of all thyroid cancers. PTC and FTC
are collectively referred to as differentiated thyroid carcinoma (DTC). Different
pathological types of thyroid cancer are characterized by significantly different
pathogenesis, biological behaviors, histological morphology, clinical manifestations,
treatment methods, and prognosis. DTC is mild in biological behaviors, with a better
prognosis. ATC is of higher malignancy, with a median survival of only 7–10 months. The
prognosis of MTC tends to be between those of the two types above.
For benign or malignant thyroid nodules that are not determined by fine needle
aspiration biopsy (FNAB), a test can be performed on fine needle aspiration specimens
for thyroid cancer molecular markers, such as BRAF mutations, Ras mutations, and RET/PTC
rearrangements. This helps improve diagnosis accuracy. Detection of BRAF mutations in
preoperative fine needle aspiration specimens is also conducive to the diagnosis and
clinical prognosis prediction of PTC and the development of individualized diagnosis and
treatments. The American Thyroid Association (ATA) recommends that all patients with
MTC, even those with a significant tendency of sporadic MTC, should be tested for RET
mutations.
References:
1.2015 American Thyroid Association Management Guidelines for Adult Patients with
Thyroid Nodules and Differentiated Thyroid Cancer.
2.Thyroid Cancer Diagnosis and Treatment Practice (2018 Version).
3.2015 American Thyroid Association Management Guidelines for Adult Patients with
Thyroid Nodules and Differentiated Thyroid Cancer.

Breast cancer is the most common type of malignant tumors in women, which seriously threatens women's health all over the world. In 2015, there were about 304,000 new cases of female breast cancer in China, with an incidence of 45.29/100,000 and about 70,000 deaths. Among new cases of breast cancer each year, about 3%–10% of women have distant metastases at the time of diagnosis. 30% of the early-stage cases can evolve into advanced breast cancer, and their five-year survival rate is only 20%. Advanced breast cancer is a special stage in breast cancer development and differs from other stages in both treatment and efficacy. Patients with advanced breast cancer face pressure from multiple aspects, including the disease itself, psychological factors, and related economic factors. About 20% of breast cancers are advanced locally, but free of distant metastases at first diagnosis. Before treatments, a needle biopsy should be performed to obtain the expression status of histological and biological markers, and gene mutations (e.g., PI3K and BRCA mutations) should be detected as soon as possible to assist with developing a treatment plan. The neoadjuvant therapy should be actively adopted for patients with locally advanced diseases that may transfer into a radical cure by surgery. For advanced breast cancers with HR-positive HER-2 negative, CDK4/6 inhibitors in combination with endocrine therapy can be selected preferentially for patients without visceral crisis. Chemotherapy may be considered for patients who are not suitable for endocrine therapy or have seen rapid disease progression after endocrine therapy. Endocrine therapy can be used as maintenance therapy after the disease is effectively controlled. Platinum chemotherapy is an important treatment option for patients with triple-negative breast cancer, patients with BRCA mutations have better efficacy. Therefore, BRCA gene tests are recommended, especially for younger patients. At present, only germline BRCA1/2 gene mutations in hereditary breast cancer are valuable for clinical application and treatment, so genetic tests should be performed as early as possible when targeted therapies are available. The NCCN guidelines recommend that the 21-gene test can be used for some hormone receptor-positive and HER2-negative patients.
References:
1.Guidelines for Standardized Diagnosis and Treatment of Advanced Breast Cancer in China
(2020 Version).
2.NCCN Guidelines Version2.2021 Invasive Breast Cancer.

Urothelial carcinoma refers to urothelial malignant tumors that occur in the renal
pelvis, ureter, bladder, and urethra. Among these, bladder cancer accounts for about 90%
of cases, with an incidence rate that ranks ninth worldwide, just following prostate
cancer, amongst other urinary system tumors. It is also one of the most expensive tumors
to treat[1]. At present, there are still some concerns with the clinical
treatment of bladder cancer in both early diagnosis and advanced treatment.
For early auxiliary diagnosis, the diagnoses and follow-up examinations of bladder
cancer mainly rely on a combination of cystoscopies examination, imaging, and testing of
urine exfoliated cells. However, cystoscopies are invasive and painful, risking
infection and bleeding. Urine exfoliative cytology has good specificity but is extremely
low in sensitivity, which can be easily disrupted by factors such as urinary tract
infection. Also, for patients with painless hematuria in clinical practice, the presence
of tumors cannot be determined by cystoscopies and imaging, and there is still no good
supplementary means to assist physicians with judgment calls. Therefore, it has become
the popular topic for research in recent years to seek noninvasive, highly sensitive,
highly specific, simple and feasible tumor markers as the basis for diagnosis and
follow-up examinations of urothelium carcinoma. At present, multiple gene mutations,
combined with the gene methylation model can effectively identify benign and malignant
bladder cancers at early stages[2].
For advanced treatments, treatment of bladder cancer in China still consist of
treatments based on systemic chemotherapy, surgical resection, radiation therapy,
targeted therapy, and immunotherapy. In recent years, with the rise of immunotherapy,
five types of PD1/PD-L1 inhibitors have been approved by the FDA as second-line
therapies. Atezolizumab and pembrolizumab can be used as the first-line therapy for
mUC(urothelium carcinoma), with no indicator for cis-platinum or with high PD-L1
expression[3]; however, there has been no improvement in targeted therapies
until April 2019, when Erdafitinib, the first targeting drug for bladder cancer, was
finally approved and marketed in the United States. Since then, the treatments for
bladder cancer has entered the era of precision therapy[4].
References:
[1] Campbell Wash Urology. (Ninth Edition).
[2] Xu, Yansheng, Ma, etc. A Urine-Based Liquid Biopsy Method for Detection of Upper
Tract Urinary Carcinoma[J]. Front Oncol. 2021 Feb 9;10:597486.
[3] Educational Book. The 23th Annual Meeting of Chinese Society of Clinical Oncology
(CSCO).
[4] Y.Loriot, A. Necchi, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial
Carcinoma[J]. 2019,381:338-348.


